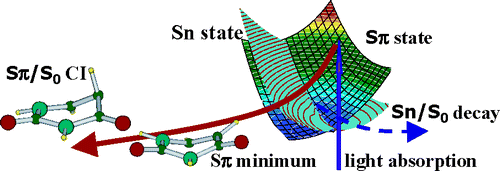
The first comprehensive quantum mechanical study of solvent effects on the behavior of the two lowest energy excited states of uracil derivatives is presented. The absorption and emission spectra of uracil and 5-fluorouracil in acetonitrile and aqueous solution have been computed at the time-dependent density-functional theory level, using the polarizable continuum model (PCM) to take into account bulk solvent effects. The computed spectra and the solvent shifts provided by our method are close to their experimental counterpart. The S0/S1 conical intersection, located in the presence of hydrogen-bonded solvent molecules by CASSCF (8/8) calculations, indicates that the mechanism of ground-state recovery, involving out-of-plane motion of the 5 substituent, does not depend on the nature of the solvent. Extensive explorations of the excited-state surfaces in the Franck−Condon (FC) region show that solvent can modulate the accessibility of an additional decay channel, involving a dark n/π* excited state. This finding provides the first unifying explanation for the experimental trend of 5-fluorouracil excited-state lifetime in different solvents. The microscopic mechanisms underlying solvent effects on the excited-state behavior of nucleobases are discussed.
•  Physics and chemistry for life sciences and the environment › Physics and life
Physics and chemistry for life sciences and the environment › Physics and life
• Service des Photons Atomes et Molécules • Service des Photons Atomes et Molécules