Céline JAUDOIN, Isabelle GRILLO, Fabrice COUSIN, Maria GEHRKE, Malika OULDALI, Ana-Andreea ARTENI, Luc PICTON, Christophe RIHOUEY, Fanny SIMELIERE, Amélie BOCHOT, Florence AGNELY
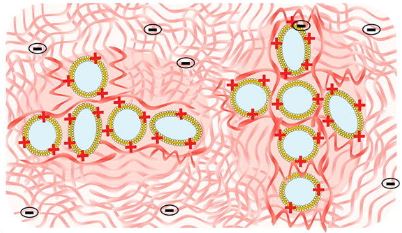
Mixtures of hyaluronic acid (HA) with liposomes lead to hybrid colloid–polymer systems with a great interest in drug delivery. However, little is known about their microstructure. Small angle neutron scattering (SANS) is a valuable tool to characterize these systems in the semi-dilute entangled regime (1.5% HA) at high liposome concentration (80 mM lipids). The objective was to elucidate the influence of liposome surface (neutral, cationic, anionic or anionic PEGylated), drug encapsulation and HA concentration in a buffer mimicking biological fluids (37 °C). First, liposomes were characterized by SANS, cryo-electron microscopy, and dynamic light scattering and HA by SANS, size exclusion chromatography, and rheology. Secondly, HA-liposome mixtures were studied by SANS. In HA, liposomes kept their integrity. Anionic and PEGylated liposomes were in close contact within dense clusters with an amorphous organization. The center-to-center distance between liposomes corresponded to twice their diameter. A depletion mechanism could explain these findings. Encapsulation of a corticoid did not modify this organization. Cationic liposomes formed less dense aggregates and were better dispersed due to their complexation with HA. Liposome surface governed the interactions and microstructure of these hybrid systems.
https://doi.org/10.1016/j.jcis.2022.07.146
Raphael Dos Santos Morais, Olivier Delalande, Javier Perez, Dominique Mias-Lucquin, Melanie Lagarrigue, Anne Martel, Anne-Elisabeth Molza, Angelique Cheron, Celine Raguenes-Nicol, Thomas Chenuel, Arnaud Bondon, Marie-Sousai Appavou, Elisabeth Le Rumeur, Sophie Combet, and Jean-Francois Hubert
Scaffolding proteins play important roles in supporting the plasma membrane (sarcolemma) of muscle cells. Among them, dystrophin strengthens the sarcolemma through protein-lipid interactions, and its absence due to gene mutations leads to the severe Duchenne muscular dystrophy. Most of the dystrophin protein consists of a central domain made of 24 spectrin-like coiled-coil repeats (R). Using small angle neutron scattering (SANS) and the contrast variation technique, we specifically probed the structure of the three first consecutive repeats 1–3 (R1–3), a part of dystrophin known to physiologically interact with membrane lipids. R1–3 free in solution was compared to its structure adopted in the presence of phospholipid-based bicelles. SANS data for the protein/lipid complexes were obtained with contrast-matched bicelles under various phospholipid compositions to probe the role of electrostatic interactions. When bound to anionic bicelles, large modifications of the protein threedimensional structure were detected, as revealed by a significant increase of the protein gyration radius from 42 5 1 to 60 5 4 A . R1–3/anionic bicelle complexes were further analyzed by coarse-grained molecular dynamics simulations. From these studies, we report an all-atom model of R1–3 that highlights the opening of the R1 coiled-coil repeat when bound to the membrane lipids. This model is totally in agreement with SANS and click chemistry/mass spectrometry data. We conclude that the sarcolemma membrane anchoring that occurs during the contraction/elongation process of muscles could be ensured by this coiled-coil opening. Therefore, understanding these structural changes may help in the design of rationalized shortened dystrophins for gene therapy. Finally, our strategy opens up new possibilities for structure determination of peripheral and integral membrane proteins not compatible with different high-resolution structural methods.
Dos Santos Morais R et al. Biophys J 115: 1231–1239, 2018.
https://doi.org/10.1016/j.bpj.2018.07.039









 Physics and chemistry for life sciences and the environment
Physics and chemistry for life sciences and the environment