Headlines 2012
|
Ionized molecules are involved in many chemical reactions, and participate for an important part in the chemistry of the upper atmosphere and interstellar clouds. Data on the vibrational spectroscopy of these ions are thus needed to better understand the dynamics and energetics of so diluted matter. Photoelectron spectroscopies are method of choice to characterize these molecules and their vibrational states, but are often ineffective when the structure of the neutral molecule is very different from that of the ion. The Laboratoire Francis Perrin (URA 2453, CEA - CNRS) in collaboration with the team of Chimie Théorique du Laboratoire Modélisation et Simulation Multi Echelle (MSME UMR 8208 CNRS, Univ Paris-Est Marne-La-Vallée) participated in the development of a new spectroscopic method to achieve the desired data, not accessible by conventional methods. |
The description of the interactions controlling the shape of a protein is crucial in understanding the cellular mechanisms, but is still difficult to achieve on biological systems because of their complexity.
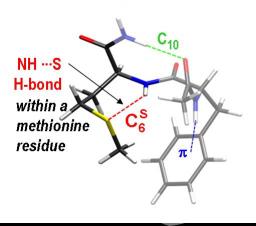
In this context, the use of model molecules makes accessible to experiments many biological problems lying at the heart of current societal issues. Gas-phase IR/UV spectroscopy of small peptides is an outstanding example. The study of peptides containing the methionine residue has recently shown that NHamide---Smethionine hydrogen bonds are particularly strong. They are, for example, as strong as the "classical" NHamide---OCamide hydrogen bonds which define the secondary structure of proteins.
Analysis of protein structures identified to date reveals that the type of NHamide---Smethionine bond observed experimentally occurs on 12% of methionines. Comparison of the parameters defining the NHamide---Smethionine bond shows that the hydrogen bonds formed in the gas phase faithfully reproduce those observed in proteins. This strongly suggests that the properties of the NHamide---Smethionine bonds highlighted in the gas phase, especially their strength, are identical to those naturally occurring in proteins.
The study of the constraints imposed to the backbone by these NHamide---Smethionine bonds shows that they reduce the range of possible values for the Ramachandran angles. This phenomenon common to gas-phase peptides and proteins having these NHamide---Smethionine bonds thus illustrates the effect that these bonds can have on the rigidity of the peptide backbone. These results already open interesting paths in understanding the way antitumor drugs act. Journal of Physical Chemistry Letters 2012, 3, 755−759