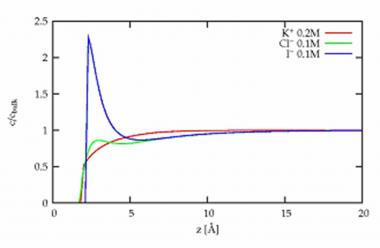
Typical profile of the ion concentrations at the surface of a mixture KCl (0,1 mole/ℓ) and KI (0,1 mole/ℓ). Red: K+ ions, green: Cl- ions and blue: I- ions.
When a salt like NaCl is dissolved in water, sodium and chlorine separate in ionic form Na+ and Cl-, become surrounded by water molecules and disperse. This deeply modifies the nature of the solvent which becomes for example, a good electricity conductor. At the surface, the distribution of the ions remains however poorly known, although many "contact" properties depend on it.
Thus, why the surface stress of water decreases with HCl whereas it increases with NaCl? Why KCl is twice more effective than NaCl to crystallize the lysozyme [1] ? Questions of this type, which illustrate the ionic specificity, abound in biology, science of the environment and the atmosphere, sciences of materials, physicochemistry… These effects, which some were described by Hofmeister in 1888 [2], are still not fully explained today. Up to now, only empirical laws were given from macroscopic observations. The difficulty holds from that these effects are primarily due to very short range (below 1 nanometer) strong couplings between the ions and the solvent molecules, and from the lack of measurements of ionic profiles at the interfaces.
By studying mixtures of salt solutions by x-ray fluorescence under grazing incidence at ESRF (synchrotron of Grenoble), it was possible to precisely determine their composition at the surface. The comparison of pure salt solutions and mixtures is here essential to right identify and measure the very short range interactions. These interactions are only responsible for the observed differences, the Coulomb interactions between the ions of same charge being unchanged. It is observed that iodides are more attracted towards the surface than the bromides which are more attracted than chlorides. Quantitatively, these measurements of the concentration profiles can be reproduced by a simple model of ionic spheres in a continuous solvent, by adding to the Coulomb interactions (ions of opposite charges attract each other) and the dispersion interaction (the solvent tends to disperse the ions), a short range interaction [3].
[1] lysozyme : protein of tears, saliva, the egg white (albumen)…
[2] F. Hofmeister, “Zur Lehre von der Wirkung der Salze II”, Arch. Exp. Pathol. Pharmakol. 25 p. 247 (1888)
[3] Osmotic coefficients and surface tensions of aqueous electrolyte solutions: Role of dispersion forces
W. Kunz, L. Belloni, O. Bernard and B. Ninham ,Journal of Physical Chemistry B 108 (2004) 2398.
Molecular structure of salt solutions: A new view of the interface with implications heterogeneous atmospheric chemistry
P. Jungwirth and D. Tobias , Journal of Physical Chemistry B 105 (2001) 10468.
Reference:
Specific Ion Adsorption and Short-Range Interactions at the Air Aqueous Solution Interface
V. Padmanabhan, J. Daillant, L. Belloni, Serge Mora, M. Alba, O. Konovalov
Phys. Rev. Lett. 99, 086105 (2007)