2011
Welcome to Complex Liquid Thermoelectrics Research group

NEW!!! Internship position (4-6 months, experimental) for thermally chargeable supercapacitor develoment and characterization
M2 level, starting Feb/March 2020. Possible continuation as a PhD candidate from Fall 2020.
To know more click HERE
Who & What
We study thermal-to-electrical energy conversion phenomena in liquids by combining experimental techniques from physics, physical chemistry and electrochemistr. It is an exciting new field of research that is attracting increased attention as an alternative solution to semi-conductor based thermoelectric materials. The underlying physics , the types of complex liquids and possible future application paths are described below.
Also....
Please visit our MAGENTA project page https://www.magenta-h2020.eu
A member of NANOUPTAKE– Overcoming Barriers to Nanofluids Market Uptake (COST Action CA15119)
Thermoelectricity at a glance
The application of a temperature difference ΔT across a solid conductor causes mobile charge carriers to diffuse from hot to cold region, giving rise to a thermoelectric voltage V = -Se∆T. The prefactor Se is called th thermopower or Seebeck coefficient since the discovery of this phenomenon by Seebeck in 1821. This enables the conversion of heat to electricity.
In ordinary metals like copper, the Seebeck coefficient Se is of the order of 10 μV/K. In the mid 20th century much higher Seebeck coefficients of a few hundreds of μV/K were obtained in low-gap semi-conductors, and those materials are still the object of intense research activities, with the perspective of converting low-grade wasted heat; e.g., body heat, car exhausts, industrial waste streams, solar heating, etc., into electric energy. Conversely, the application of voltage ΔV results in a temperature difference (the Peltier effect) such that the same material can be used as a thermoelectric (TE)-cooler.
Despite the robustness of TE-technology (including long life-time, no moving parts, etc.) and its wide range of applicability (wherever a thermal gradient exists), the TE-generators market has so far been restricted to a small number of technological areas due to their low efficiency. The thermal-to-electrical energy conversion efficiency, η of a given TE device is most often expressed as a function of a dimensionless parameter ZT, called “figure of merit”:
ZT = TSe2(σ/κ) (1).
ZT is proportional to the square of the Seebeck coefficient Se and to the ratio between the electrical (σ) and thermal (κ) conductivities. Up until 20 years ago, TE-devices had been based almost exclusively on low-gap solid-state bulk semiconductors whose ZT values are comprised in the ~0.1 – 1.0 range [1]. Compared to other heat engines (see Figure 1), there is no wonder why the TE-technology was not considered for a wider and larger-scale heat recovery applications.
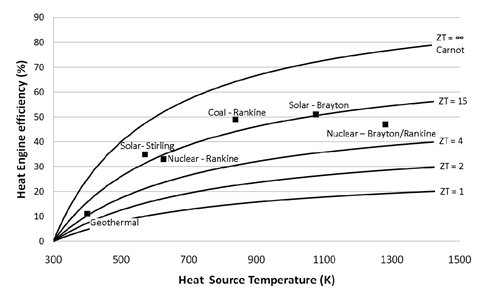
Figure 1: TE-conversion efficiency as a function of ZT and heat source temperature compared to other heat engine technologies. Image taken from [2]
In the mid-1990’s Hicks et al., [2] predicted significant improvements in the thermoelectric efficiency in nanoscale elements. This has generated a great surge in nanostructured thermoelectric (micro)generators (See figure (left)). In a very simplified term, by ‘nano’structuring, one can reduce the material’s thermal conductivity κ, without affecting its electrical conductivity σ, resulting in an enhancement of ZT, figure of merit (see Eq. (1)).
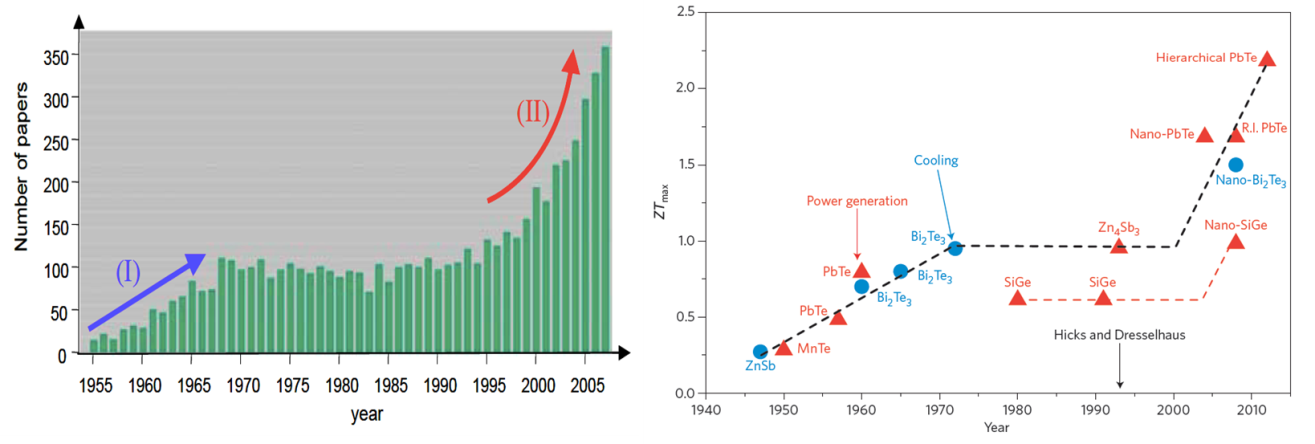
Figure 2: (Left) Publication trend in thermoelectric research [3] and (Right) Historical progress in ZT values [4].
High ZT values (over 2 at room temperature, see Figure 2 right panel) made of bismuth-telluride and its alloys have been reported. Unfortunately, these nanostructured devices are limited to very small active surface areas and incur huge production costs. Furthermore nearly all high-performance TE materials contain rare and often toxic raw materials (high scarcity and health risks) [5], highlighting a need for a transformation thermoelectric technology made with less critical material.
Thermoelectric effects in liquid electrolytes
Liquid electrolytes are characterised by the presence of several ion species (dissolved in a liquid matrix) and their "thermoelectric" effects have been known since the end of the 19th century. The observed TE-coefficients in electrolytes are generally an order of magnitude larger that the semiconductor counterparts, including nanostructured materials (see Figure 3 below). The electrical conductivity of these liquids, however, is a few orders of magnitude lower than solid counterparts and therefore, liquid based TE-systems have long been considered technologically irrelevant despite their advantages; e.g., material abundance, low production costs, etc..
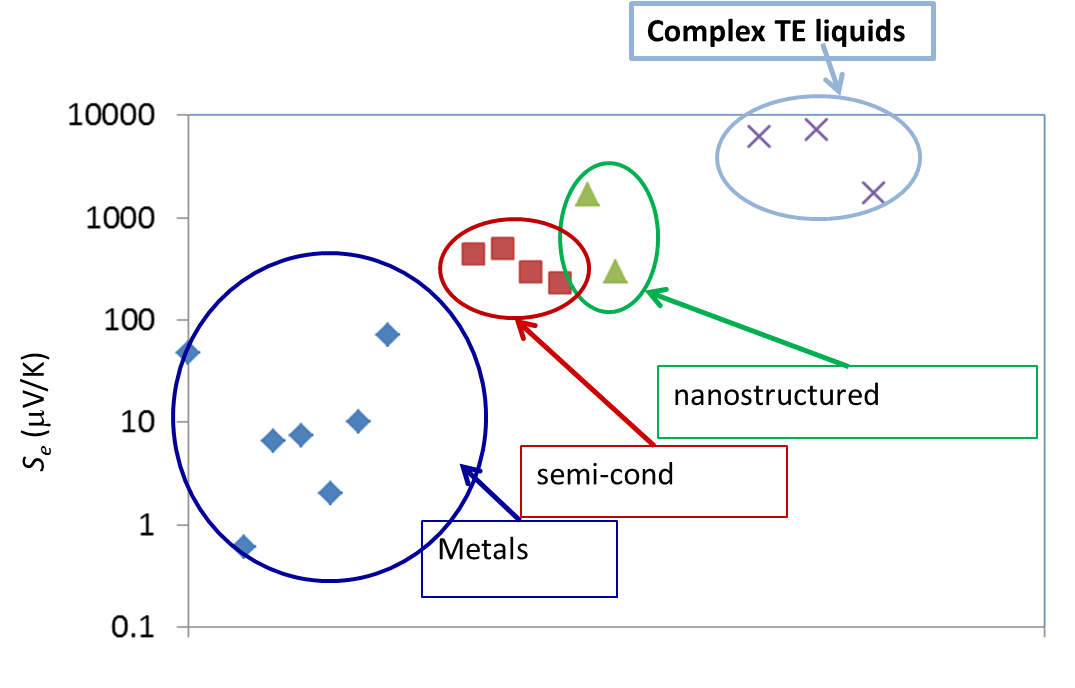
Figure 3: Comparison of TE voltage (open circuit) of different materials
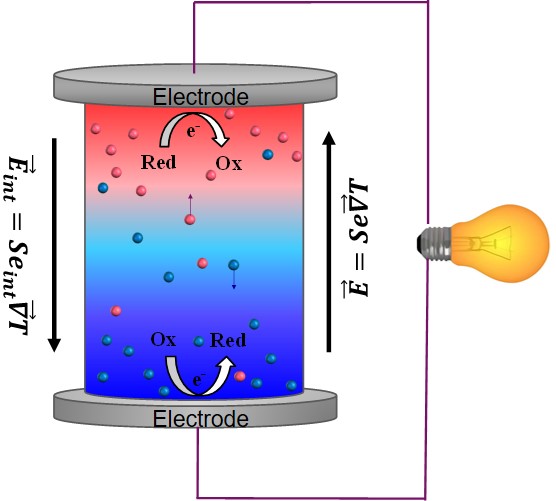
Since the discovery of TE-effects in complex liquids such as ionic-liquids/solvent binary mixtures, and charged colloidal and macromolecular suspensions, such perception surrounding liquid thermoelectrics is changing in recent years. Broadly speaking, there are 3 different sources of thermoelectric power in electrolyte containing thermoelectric cells; namely, (a) Thermogalvanic effect b) thermodiffusion of ionic species and c) Selective ion attachments of ionic species to the hot and cold electrodes.
- Thermogalvanic effect: Also known as 'thermoelectrochemical effect,’ relies on redox molecules to reversibly exchange electrons (oxidation-reduction mechanism) with hot-and cold electrodes to produce large reaction entropy change; i.e., thermogalvanic-voltage. The redox couples not only serve to produce ‘voltage’ but are responsible for producing electric current in a thermocells by exchanging electrons with hot and cold electrodes. Typically observed Seebeck coefficient in thermogalvanic cells containing ILs and other liquid electrolytes is about 1 mV/K an order of magnitude larger that the semiconductor counterparts, including nanostructured materials.
- Thermodiffusion effect: The TE-effect in complex liquids (electrolytes, ionic liquids, colloidal solutions etc.) is further coupled to the movement of dissolved charged species (ions, molecules or colloidal particles) and is closely related to the “Soret effect” which describes the concentration gradients, ni induced by a temperature gradient; (Δni/ni) = αiΔT, where a is called “Soret coefficient.” The Soret effect or “thermophoresis” has been extensively studied in the last decades [16-18] and is often used for microfluidic characterization and separation. The Soret coefficient, a, is generally proportional to the entropy transported by the moving molecules or particles (i.e., internal degrees of freedom, large solvation shell, particle/molecule size, etc.). Therefore, large thermoelectric coefficients can be expected in complex liquids exhibiting large values of .
- Electronic double layer (EDL): Closely related to the thermodiffusion effect described above, the assymetric distribution of ionic species at the hot and cold electrodes can result in the temperature dependent electronic double layer capacitance at the liquid/electrode interfaces.
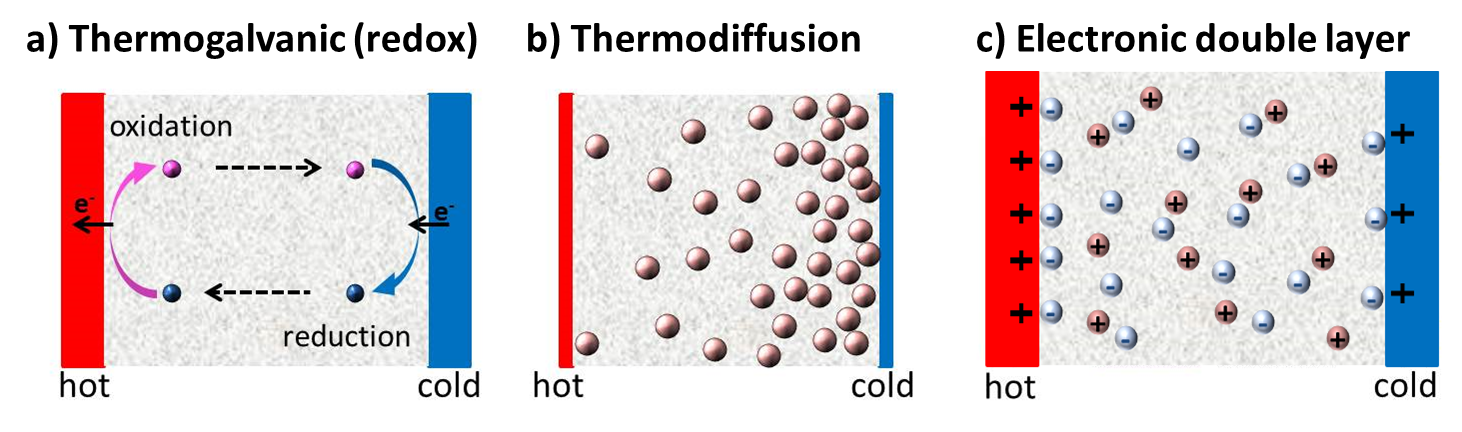
Figure 4: Different mechanisms at the origin of thermoelectric voltage in liquid electrolytes.
While these effects are independently known to exist in liquid electrolytes and complex liquids (such as colloidal suspensions and ionic liquids), the interdependency among thermogalvanic-thermodiffusion-EDL effect is still in the basic research phase. At SPHYNX, we are exploring these novel thermoelectric phenomena in various complex liquids.
We focus on ....
- Non-aqueous electrolytes: We have measured thermoelectric power in various non-aqueous electrolytes (alocohols, organic solvents) where TE voltage as high as 7mV/K has been measured [7]
- Ionic liquids: We investigate all 3 TE effects either in TE-generator or in TE-capacitor mode. [8, 9]
- Ferrofluids: In 2015, we have reported a first-of-its-kind experimental study on the coupled thermodiffusion and thermoelectric effects in ferrofluids based on organic solvents [10] and on water [11]. This work served as a true proof-of-principle, demonstrating the contribution of thermodiffusion of charged colloidal particles to the total thermoelectric voltage of the carrier fluid. Experimental parameters such as magnetic volume fraction, particle size, applied magnetic field strength and its direction that can be tuned to control the TE property of ferrofluids. The magnetic nature of particles further encourages the use of ferrofluids for magneto-thermoelectric energy conversion applications.
- Develop novel thermoelectric measurement techniques at high (above 150°C) and low (below 0°C) temperatures and under magnetic field.
|
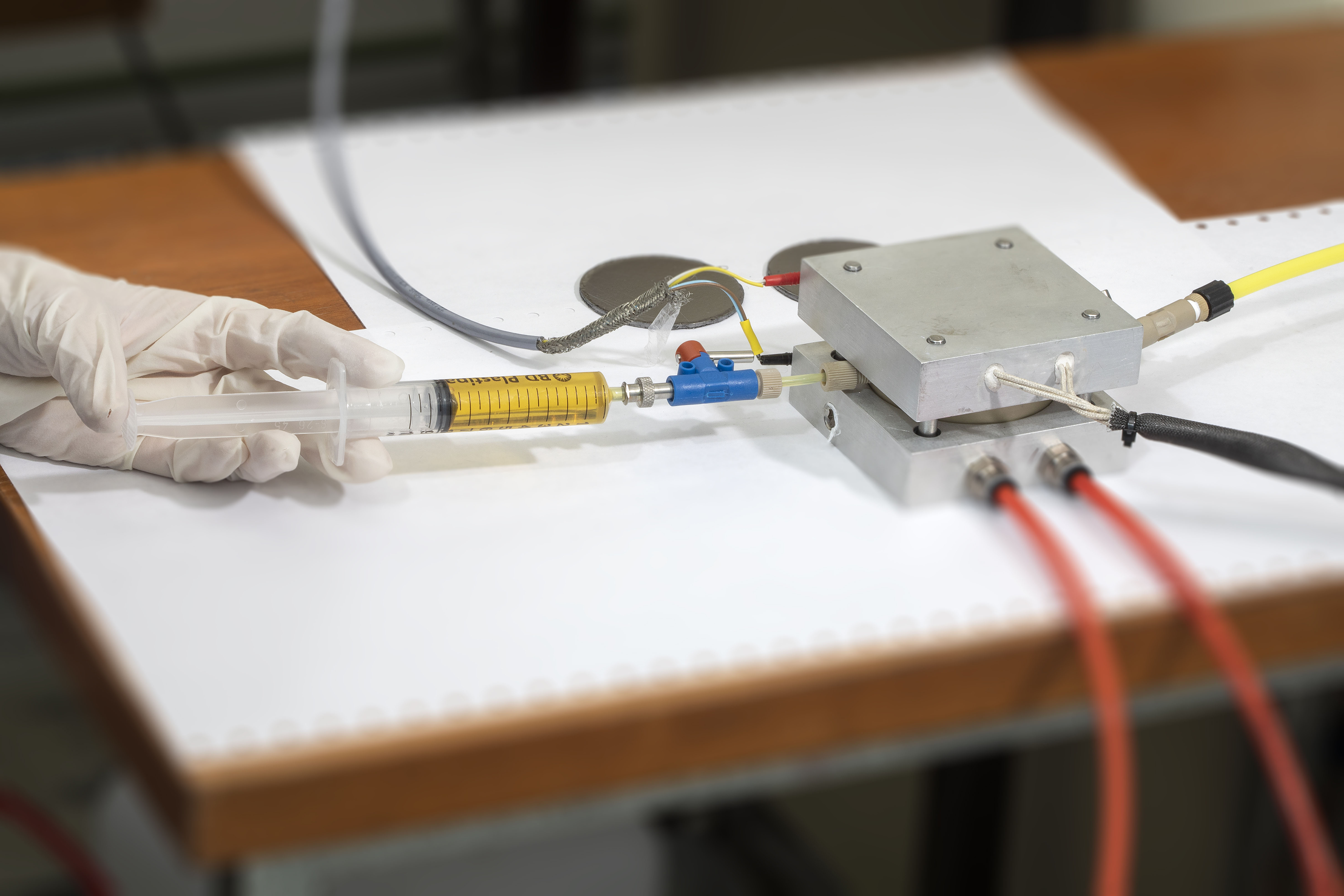
Mock-up TE-cell (by CTECH Innovation in MAGENTA) |
Simplified TE-cell |
High-temperature cell |
Electrical conductivity cell |
Medical imaging is an invaluable tool for the diagnosis of cancer especially when non-invasive techniques based on nuclear magnetic resonance are used. Their high spatial resolution stems from metal-based contrast agents which are mostly Gd(III) chelates. In spite of the prevalence of these paramagnetic contrast agents in oncology, improvements are awaited regarding their efficiency, their selectivity, and even their toxicity. Both experimental and theoretical chemists can contribute to understand the mechanism of their enhancing power and to suggest innovative contrast agents that will replace the existing ones.
Presently, atomic scale simulations of metal complexes in solution such as Gd-based contrast agents can essentially be divided in high-level quantum chemistry calculations or molecular dynamics simulations. More recent theoretical approaches could be considered as the “best of both worlds”. These are ab initio simulations launched by Car and Parrinello in 1985 but applied much later for chemistry applications. The study of Gd chelates in aqueous solution (namely ProHance®), clinically approved as MRI contrast agents, using ab initio molecular dynamics (AIMD) method was first reported by our team in 2007 [1].
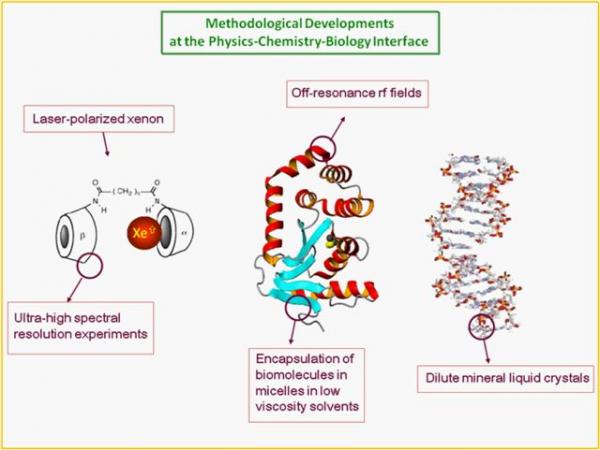
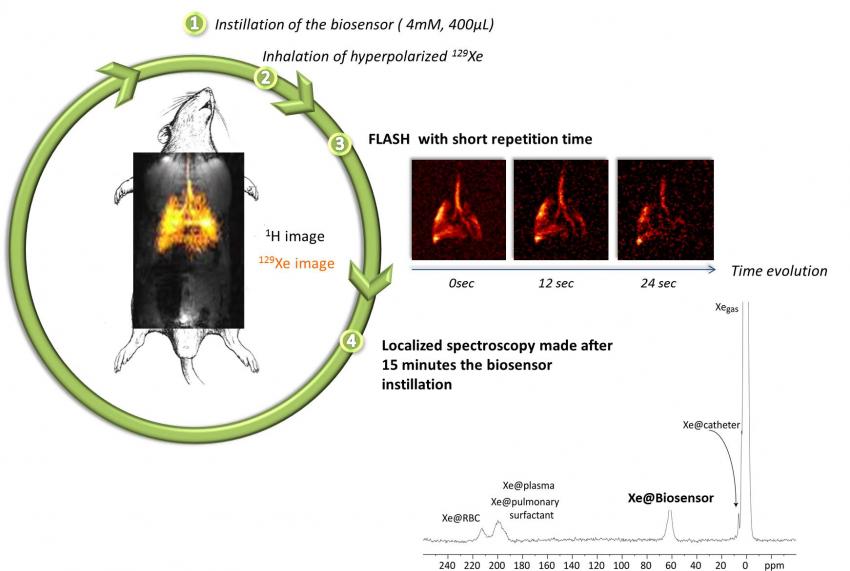
In collaboration with chemists from ENS Lyon and from CEA/iBiTeC-S and CBM Orléans, MRI experiments on living rodents are undertaken. 129Xe NMR-based biosensors designed to target a pulmonary disease are instilled in the lungs of the anesthetized animal. The acquisition is then performed just after the introduction of hyperpolarized xenon, which can occur many times and several tens of minutes after the biosensor instillation.
Page under construction
Welcome to Complex Liquid Thermoelectrics Research group

NEW!!! Internship position (4-6 months, experimental) for thermally chargeable supercapacitor develoment and characterization
M2 level, starting Feb/March 2020. Possible continuation as a PhD candidate from Fall 2020.
To know more click HERE
Who & What
We study thermal-to-electrical energy conversion phenomena in liquids by combining experimental techniques from physics, physical chemistry and electrochemistr. It is an exciting new field of research that is attracting increased attention as an alternative solution to semi-conductor based thermoelectric materials. The underlying physics , the types of complex liquids and possible future application paths are described below.
Also....
Please visit our MAGENTA project page https://www.magenta-h2020.eu
A member of NANOUPTAKE– Overcoming Barriers to Nanofluids Market Uptake (COST Action CA15119)
Thermoelectricity at a glance
The application of a temperature difference ΔT across a solid conductor causes mobile charge carriers to diffuse from hot to cold region, giving rise to a thermoelectric voltage V = -Se∆T. The prefactor Se is called th thermopower or Seebeck coefficient since the discovery of this phenomenon by Seebeck in 1821. This enables the conversion of heat to electricity.
In ordinary metals like copper, the Seebeck coefficient Se is of the order of 10 μV/K. In the mid 20th century much higher Seebeck coefficients of a few hundreds of μV/K were obtained in low-gap semi-conductors, and those materials are still the object of intense research activities, with the perspective of converting low-grade wasted heat; e.g., body heat, car exhausts, industrial waste streams, solar heating, etc., into electric energy. Conversely, the application of voltage ΔV results in a temperature difference (the Peltier effect) such that the same material can be used as a thermoelectric (TE)-cooler.
Despite the robustness of TE-technology (including long life-time, no moving parts, etc.) and its wide range of applicability (wherever a thermal gradient exists), the TE-generators market has so far been restricted to a small number of technological areas due to their low efficiency. The thermal-to-electrical energy conversion efficiency, η of a given TE device is most often expressed as a function of a dimensionless parameter ZT, called “figure of merit”:
ZT = TSe2(σ/κ) (1).
ZT is proportional to the square of the Seebeck coefficient Se and to the ratio between the electrical (σ) and thermal (κ) conductivities. Up until 20 years ago, TE-devices had been based almost exclusively on low-gap solid-state bulk semiconductors whose ZT values are comprised in the ~0.1 – 1.0 range [1]. Compared to other heat engines (see Figure 1), there is no wonder why the TE-technology was not considered for a wider and larger-scale heat recovery applications.

Figure 1: TE-conversion efficiency as a function of ZT and heat source temperature compared to other heat engine technologies. Image taken from [2]
In the mid-1990’s Hicks et al., [2] predicted significant improvements in the thermoelectric efficiency in nanoscale elements. This has generated a great surge in nanostructured thermoelectric (micro)generators (See figure (left)). In a very simplified term, by ‘nano’structuring, one can reduce the material’s thermal conductivity κ, without affecting its electrical conductivity σ, resulting in an enhancement of ZT, figure of merit (see Eq. (1)).

Figure 2: (Left) Publication trend in thermoelectric research [3] and (Right) Historical progress in ZT values [4].
High ZT values (over 2 at room temperature, see Figure 2 right panel) made of bismuth-telluride and its alloys have been reported. Unfortunately, these nanostructured devices are limited to very small active surface areas and incur huge production costs. Furthermore nearly all high-performance TE materials contain rare and often toxic raw materials (high scarcity and health risks) [5], highlighting a need for a transformation thermoelectric technology made with less critical material.
Thermoelectric effects in liquid electrolytes
Liquid electrolytes are characterised by the presence of several ion species (dissolved in a liquid matrix) and their "thermoelectric" effects have been known since the end of the 19th century. The observed TE-coefficients in electrolytes are generally an order of magnitude larger that the semiconductor counterparts, including nanostructured materials (see Figure 3 below). The electrical conductivity of these liquids, however, is a few orders of magnitude lower than solid counterparts and therefore, liquid based TE-systems have long been considered technologically irrelevant despite their advantages; e.g., material abundance, low production costs, etc..

Figure 3: Comparison of TE voltage (open circuit) of different materials
Since the discovery of TE-effects in complex liquids such as ionic-liquids/solvent binary mixtures, and charged colloidal and macromolecular suspensions, such perception surrounding liquid thermoelectrics is changing in recent years. Broadly speaking, there are 3 different sources of thermoelectric power in electrolyte containing thermoelectric cells; namely, (a) Thermogalvanic effect b) thermodiffusion of ionic species and c) Selective ion attachments of ionic species to the hot and cold electrodes.
- Thermogalvanic effect: Also known as 'thermoelectrochemical effect,’ relies on redox molecules to reversibly exchange electrons (oxidation-reduction mechanism) with hot-and cold electrodes to produce large reaction entropy change; i.e., thermogalvanic-voltage. The redox couples not only serve to produce ‘voltage’ but are responsible for producing electric current in a thermocells by exchanging electrons with hot and cold electrodes. Typically observed Seebeck coefficient in thermogalvanic cells containing ILs and other liquid electrolytes is about 1 mV/K an order of magnitude larger that the semiconductor counterparts, including nanostructured materials.
- Thermodiffusion effect: The TE-effect in complex liquids (electrolytes, ionic liquids, colloidal solutions etc.) is further coupled to the movement of dissolved charged species (ions, molecules or colloidal particles) and is closely related to the “Soret effect” which describes the concentration gradients, ni induced by a temperature gradient; (Δni/ni) = αiΔT, where a is called “Soret coefficient.” The Soret effect or “thermophoresis” has been extensively studied in the last decades [16-18] and is often used for microfluidic characterization and separation. The Soret coefficient, a, is generally proportional to the entropy transported by the moving molecules or particles (i.e., internal degrees of freedom, large solvation shell, particle/molecule size, etc.). Therefore, large thermoelectric coefficients can be expected in complex liquids exhibiting large values of .
- Electronic double layer (EDL): Closely related to the thermodiffusion effect described above, the assymetric distribution of ionic species at the hot and cold electrodes can result in the temperature dependent electronic double layer capacitance at the liquid/electrode interfaces.

Figure 4: Different mechanisms at the origin of thermoelectric voltage in liquid electrolytes.
While these effects are independently known to exist in liquid electrolytes and complex liquids (such as colloidal suspensions and ionic liquids), the interdependency among thermogalvanic-thermodiffusion-EDL effect is still in the basic research phase. At SPHYNX, we are exploring these novel thermoelectric phenomena in various complex liquids.
We focus on ....
- Non-aqueous electrolytes: We have measured thermoelectric power in various non-aqueous electrolytes (alocohols, organic solvents) where TE voltage as high as 7mV/K has been measured [7]
- Ionic liquids: We investigate all 3 TE effects either in TE-generator or in TE-capacitor mode. [8, 9]
- Ferrofluids: In 2015, we have reported a first-of-its-kind experimental study on the coupled thermodiffusion and thermoelectric effects in ferrofluids based on organic solvents [10] and on water [11]. This work served as a true proof-of-principle, demonstrating the contribution of thermodiffusion of charged colloidal particles to the total thermoelectric voltage of the carrier fluid. Experimental parameters such as magnetic volume fraction, particle size, applied magnetic field strength and its direction that can be tuned to control the TE property of ferrofluids. The magnetic nature of particles further encourages the use of ferrofluids for magneto-thermoelectric energy conversion applications.
- Develop novel thermoelectric measurement techniques at high (above 150°C) and low (below 0°C) temperatures and under magnetic field.
|
Mock-up TE-cell (by CTECH Innovation in MAGENTA) |
Simplified TE-cell |
High-temperature cell |
Electrical conductivity cell |
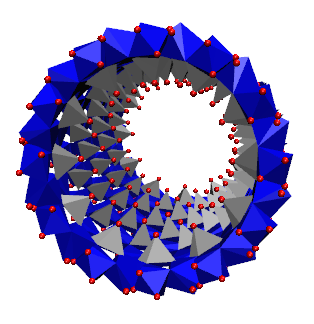
short imogolite nanotube.
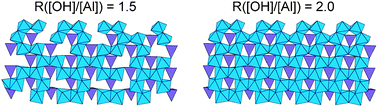
Depuis la découverte des nanotubes de carbone (NT), la synthèse et la caractérisation de structures de forme similaire comme les nanotubes inorganiques, les nanotiges ou les nanofils suscitent un grand intérêt. Cependant, des limitations telles que la pureté, la complexité du protocole de synthèse, le faible rendement de production ou la large dispersion de taille restent des obstacles majeurs pour les applications à l'échelle industrielle.
Dans ce contexte, les imogolites synthétiques apparaissent comme une exception. Ce sont des NT d'aluminosilicates à paroi simple de 2 nm de diamètre et jusqu'à 1 μm de longueur, de formule empirique (OH)3Al2O3Si OH, dont la structure a été déterminée par diffraction des rayons X (XRD), résonance magnétique nucléaire (RMN) à l'état solide et microscopie électronique à transmission (MET). Des analogues de l'imogolite, où les atomes de Ge remplacent ceux de Si ont été synthétisés avec succès. Les premiers rapports sur leur synthèse indiquent des conditions très diluées (c'est-à-dire millimolaires). Cependant des analogues d'imogolite ont récemment été obtenus à partir de solutions 100 fois plus concentrées, ouvrant ainsi la voie aux applications à grande échelle. Ces analogues ont été décrits comme étant structurellement identiques à l'imogolite naturelle Al-Si, masi avec un diamètre de tube supérieur (∼3.3 nm) et des longueurs plus courtes (inférieures à 100 nm). Au laboratoire LIONS, le mécanisme de formation de l'imogolite et des analogues de l'imogolite a été largement étudié ainsi que leur utilisation dans un nouveau matériau auto-assemblé.
Since the discovery of carbon nanotubes (NTs), there is great interest in the synthesis and characterization of similar shaped structures like inorganic nanotubes, nanorods, or nanowires. However, limitations such as purity, complexity of the synthesis protocol, low-yield production, and wide size polydispersity still remain major impediments for industrial-scale applications.
In this context, synthetic imogolites appear as an exception. They are singlewalled aluminosilicate NTs of 2 nm diameter and up to 1 μm in length with the empirical formula (OH)3Al2O3Si OH whose structure has been determined using X-ray Diffraction (XRD), solid state Nuclear Magnetic Resonance (NMR), and Transmission Electron Microscopy (TEM). Imogolite analogues where Ge replace Si atoms have been succesfully synthesized. Although early reports of their synthesis involved diluted (i.e., millimolar) conditions, these imogolite analogues were recently obtained from 100 times more concentrated solutions, thereby opening the route for large scale applications. These analogues have been described to be structurally identical to the Al-Si imogolite, except for a larger tube diameter (∼3.3 nm) and shorter length (less than 100 nm). We areng the imogolite and Imogolite analogue formation mechanism. We are also studying the use of Imogolite and Imogolite analogue in self assembled new material.
Contact : Antoine Thill (NIMBE/LIONS)
Medical imaging is an invaluable tool for the diagnosis of cancer especially when non-invasive techniques based on nuclear magnetic resonance are used. Their high spatial resolution stems from metal-based contrast agents which are mostly Gd(III) chelates. In spite of the prevalence of these paramagnetic contrast agents in oncology, improvements are awaited regarding their efficiency, their selectivity, and even their toxicity. Both experimental and theoretical chemists can contribute to understand the mechanism of their enhancing power and to suggest innovative contrast agents that will replace the existing ones.
Presently, atomic scale simulations of metal complexes in solution such as Gd-based contrast agents can essentially be divided in high-level quantum chemistry calculations or molecular dynamics simulations. More recent theoretical approaches could be considered as the “best of both worlds”. These are ab initio simulations launched by Car and Parrinello in 1985 but applied much later for chemistry applications. The study of Gd chelates in aqueous solution (namely ProHance®), clinically approved as MRI contrast agents, using ab initio molecular dynamics (AIMD) method was first reported by our team in 2007 [1].
The Laboratory 'Structure and Dynamics by Magnetic Resonance' (LSDRM) belongs to NIMBE, UMR CEA/CNRS 3685.
The research axes are centered on the conception and the use of new NMR tools. Cutting edge methods and original approaches are proposed, from instrumental developments to molecular simulations. The applications cover a large field from the gas to the solid phase, from spectroscopy to imaging, for a better knowledge of the fine structure of materials such as nuclear glasses or biological macromolecules. A strong support in Quantum Chemistry is required for an in-depth study of these different fields.
Research themes
In collaboration with chemists from ENS Lyon and from CEA/iBiTeC-S and CBM Orléans, MRI experiments on living rodents are undertaken. 129Xe NMR-based biosensors designed to target a pulmonary disease are instilled in the lungs of the anesthetized animal. The acquisition is then performed just after the introduction of hyperpolarized xenon, which can occur many times and several tens of minutes after the biosensor instillation.
2007 - today
- Hyperpolarized species for NMR/MRI
- Interatomic distance measurement using 3H solid-state NMR
- Developments for metabolomics
1999 - 2007
Welcome to Complex Liquid Thermoelectrics Research group

NEW!!! Internship position (4-6 months, experimental) for thermally chargeable supercapacitor develoment and characterization
M2 level, starting Feb/March 2020. Possible continuation as a PhD candidate from Fall 2020.
To know more click HERE
Who & What
We study thermal-to-electrical energy conversion phenomena in liquids by combining experimental techniques from physics, physical chemistry and electrochemistr. It is an exciting new field of research that is attracting increased attention as an alternative solution to semi-conductor based thermoelectric materials. The underlying physics , the types of complex liquids and possible future application paths are described below.
Also....
Please visit our MAGENTA project page https://www.magenta-h2020.eu
A member of NANOUPTAKE– Overcoming Barriers to Nanofluids Market Uptake (COST Action CA15119)
Thermoelectricity at a glance
The application of a temperature difference ΔT across a solid conductor causes mobile charge carriers to diffuse from hot to cold region, giving rise to a thermoelectric voltage V = -Se∆T. The prefactor Se is called th thermopower or Seebeck coefficient since the discovery of this phenomenon by Seebeck in 1821. This enables the conversion of heat to electricity.
In ordinary metals like copper, the Seebeck coefficient Se is of the order of 10 μV/K. In the mid 20th century much higher Seebeck coefficients of a few hundreds of μV/K were obtained in low-gap semi-conductors, and those materials are still the object of intense research activities, with the perspective of converting low-grade wasted heat; e.g., body heat, car exhausts, industrial waste streams, solar heating, etc., into electric energy. Conversely, the application of voltage ΔV results in a temperature difference (the Peltier effect) such that the same material can be used as a thermoelectric (TE)-cooler.
Despite the robustness of TE-technology (including long life-time, no moving parts, etc.) and its wide range of applicability (wherever a thermal gradient exists), the TE-generators market has so far been restricted to a small number of technological areas due to their low efficiency. The thermal-to-electrical energy conversion efficiency, η of a given TE device is most often expressed as a function of a dimensionless parameter ZT, called “figure of merit”:
ZT = TSe2(σ/κ) (1).
ZT is proportional to the square of the Seebeck coefficient Se and to the ratio between the electrical (σ) and thermal (κ) conductivities. Up until 20 years ago, TE-devices had been based almost exclusively on low-gap solid-state bulk semiconductors whose ZT values are comprised in the ~0.1 – 1.0 range [1]. Compared to other heat engines (see Figure 1), there is no wonder why the TE-technology was not considered for a wider and larger-scale heat recovery applications.

Figure 1: TE-conversion efficiency as a function of ZT and heat source temperature compared to other heat engine technologies. Image taken from [2]
In the mid-1990’s Hicks et al., [2] predicted significant improvements in the thermoelectric efficiency in nanoscale elements. This has generated a great surge in nanostructured thermoelectric (micro)generators (See figure (left)). In a very simplified term, by ‘nano’structuring, one can reduce the material’s thermal conductivity κ, without affecting its electrical conductivity σ, resulting in an enhancement of ZT, figure of merit (see Eq. (1)).

Figure 2: (Left) Publication trend in thermoelectric research [3] and (Right) Historical progress in ZT values [4].
High ZT values (over 2 at room temperature, see Figure 2 right panel) made of bismuth-telluride and its alloys have been reported. Unfortunately, these nanostructured devices are limited to very small active surface areas and incur huge production costs. Furthermore nearly all high-performance TE materials contain rare and often toxic raw materials (high scarcity and health risks) [5], highlighting a need for a transformation thermoelectric technology made with less critical material.
Thermoelectric effects in liquid electrolytes
Liquid electrolytes are characterised by the presence of several ion species (dissolved in a liquid matrix) and their "thermoelectric" effects have been known since the end of the 19th century. The observed TE-coefficients in electrolytes are generally an order of magnitude larger that the semiconductor counterparts, including nanostructured materials (see Figure 3 below). The electrical conductivity of these liquids, however, is a few orders of magnitude lower than solid counterparts and therefore, liquid based TE-systems have long been considered technologically irrelevant despite their advantages; e.g., material abundance, low production costs, etc..

Figure 3: Comparison of TE voltage (open circuit) of different materials
Since the discovery of TE-effects in complex liquids such as ionic-liquids/solvent binary mixtures, and charged colloidal and macromolecular suspensions, such perception surrounding liquid thermoelectrics is changing in recent years. Broadly speaking, there are 3 different sources of thermoelectric power in electrolyte containing thermoelectric cells; namely, (a) Thermogalvanic effect b) thermodiffusion of ionic species and c) Selective ion attachments of ionic species to the hot and cold electrodes.
- Thermogalvanic effect: Also known as 'thermoelectrochemical effect,’ relies on redox molecules to reversibly exchange electrons (oxidation-reduction mechanism) with hot-and cold electrodes to produce large reaction entropy change; i.e., thermogalvanic-voltage. The redox couples not only serve to produce ‘voltage’ but are responsible for producing electric current in a thermocells by exchanging electrons with hot and cold electrodes. Typically observed Seebeck coefficient in thermogalvanic cells containing ILs and other liquid electrolytes is about 1 mV/K an order of magnitude larger that the semiconductor counterparts, including nanostructured materials.
- Thermodiffusion effect: The TE-effect in complex liquids (electrolytes, ionic liquids, colloidal solutions etc.) is further coupled to the movement of dissolved charged species (ions, molecules or colloidal particles) and is closely related to the “Soret effect” which describes the concentration gradients, ni induced by a temperature gradient; (Δni/ni) = αiΔT, where a is called “Soret coefficient.” The Soret effect or “thermophoresis” has been extensively studied in the last decades [16-18] and is often used for microfluidic characterization and separation. The Soret coefficient, a, is generally proportional to the entropy transported by the moving molecules or particles (i.e., internal degrees of freedom, large solvation shell, particle/molecule size, etc.). Therefore, large thermoelectric coefficients can be expected in complex liquids exhibiting large values of .
- Electronic double layer (EDL): Closely related to the thermodiffusion effect described above, the assymetric distribution of ionic species at the hot and cold electrodes can result in the temperature dependent electronic double layer capacitance at the liquid/electrode interfaces.

Figure 4: Different mechanisms at the origin of thermoelectric voltage in liquid electrolytes.
While these effects are independently known to exist in liquid electrolytes and complex liquids (such as colloidal suspensions and ionic liquids), the interdependency among thermogalvanic-thermodiffusion-EDL effect is still in the basic research phase. At SPHYNX, we are exploring these novel thermoelectric phenomena in various complex liquids.
We focus on ....
- Non-aqueous electrolytes: We have measured thermoelectric power in various non-aqueous electrolytes (alocohols, organic solvents) where TE voltage as high as 7mV/K has been measured [7]
- Ionic liquids: We investigate all 3 TE effects either in TE-generator or in TE-capacitor mode. [8, 9]
- Ferrofluids: In 2015, we have reported a first-of-its-kind experimental study on the coupled thermodiffusion and thermoelectric effects in ferrofluids based on organic solvents [10] and on water [11]. This work served as a true proof-of-principle, demonstrating the contribution of thermodiffusion of charged colloidal particles to the total thermoelectric voltage of the carrier fluid. Experimental parameters such as magnetic volume fraction, particle size, applied magnetic field strength and its direction that can be tuned to control the TE property of ferrofluids. The magnetic nature of particles further encourages the use of ferrofluids for magneto-thermoelectric energy conversion applications.
- Develop novel thermoelectric measurement techniques at high (above 150°C) and low (below 0°C) temperatures and under magnetic field.
|
Mock-up TE-cell (by CTECH Innovation in MAGENTA) |
Simplified TE-cell |
High-temperature cell |
Electrical conductivity cell |
The Laboratory 'Structure and Dynamics by Magnetic Resonance' (LSDRM) belongs to NIMBE, UMR CEA/CNRS 3685.
The research axes are centered on the conception and the use of new NMR tools. Cutting edge methods and original approaches are proposed, from instrumental developments to molecular simulations. The applications cover a large field from the gas to the solid phase, from spectroscopy to imaging, for a better knowledge of the fine structure of materials such as nuclear glasses or biological macromolecules. A strong support in Quantum Chemistry is required for an in-depth study of these different fields.
Research themes
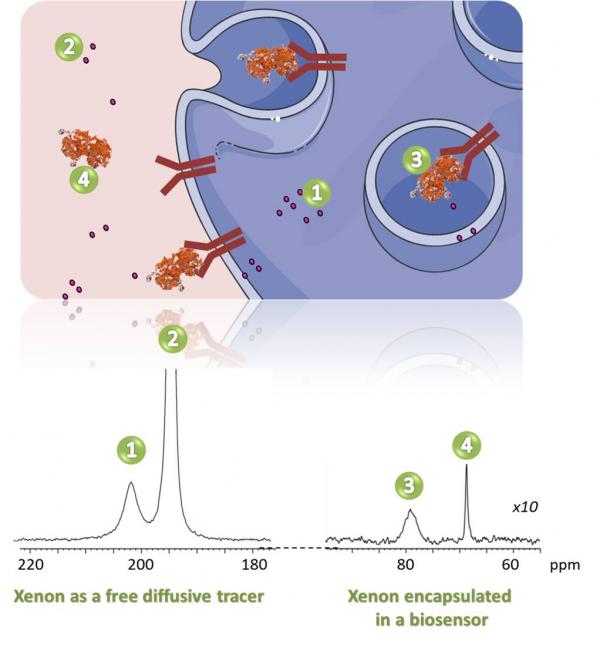
Le 129Xe hyperpolarisé peut être utilisé dans des suspensions cellulaires selon deux stratégies différentes :
- Seul, en tant que traceur diffusif libre. Le xénon pénètre dans les cellules en ~30 ms sans perdre son hyperpolarisation. L'interaction du xénon avec les cellules vivantes donne lieu à un signal RMN spécifique dans la région des 200 ppm. Le déplacement chimique et l'intensité de ce signal sont corrélés à la nature de la lignée cellulaire, à la viabilité et à la densité.
- Encapsulé dans une molécule hôte pour détecter un mécanisme biologique spécifique, par exemple le processus d'internalisation de la transferrine. Le biocapteur a été obtenu en greffant des parties de cryptophane à la transferrine.
Hyperpolarized 129Xe can be used in cell suspensions using two different strategies:
- Alone, as a free diffusive tracer. Xenon enters in the cells in ~30 ms without losing its hyper-polarization. Xenon interacting with living cells gives rise to a specific NMR signal in the 200 ppm region. The chemical shift and the intensity of this signal are correlated to the nature of the cell line, the viability and density.
- Encapsulated in a host molecule to detect a specific biological mechanism, for instance, the internalization process of transferrin. The biosensor has been obtained by the grafting of cryptophane moieties to the transferrin.
2007 - today
- Hyperpolarized species for NMR/MRI
- Interatomic distance measurement using 3H solid-state NMR
- Developments for metabolomics
1999 - 2007
Production
-
Animation for PC (S. Jubera)
-
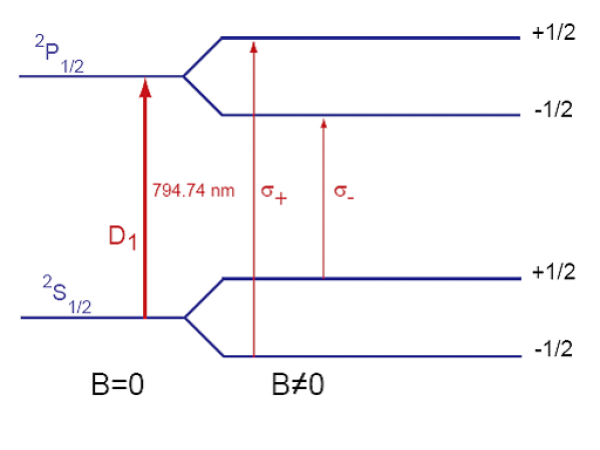
Two optical pumping setups have been built:
-
the first one based on a Titanium:Sapphire laser
-
the second one using laser diodes
-
High resolution NMR fails in the case of large macromolecules (molecular size >35 kDa) due to slow tumbling which results in short relaxation time T2 and signal loss during pulse sequences, even for proteins enriched in stable isotopes (15N, 13C, 2H), and in spite of the experiments using cross correlation effects (TROSY, CRINEPT).














 Version française
Version française