AMYLOID
CRYSTALLIZATION AT INTERFACES
Recent
studies carried in our group deal with amyloid-like peptides
whose importance in neuro-degenerative illnesses is recognized for a
long time. Such peptides form extended β-sheets domains in bulk
(amyloid fibrils) but also at an air-water interface where they can
crystallize under the form of β-sheets of large coherence length which
are also nicely ordered in the direction perpendicular to the hydrogen
bonds [1]. These peptides were selected because β-sheets domains can be
used as templates for biomimetic mineralization studies in order to
model the growth of biominerals like nacre shells. These shells are
nanocomposites where proteins including β-sheets domains are believed
to nucleate and organize the calcium carbonate mineral phase.
This thesis work will be dedicated to elucidate such crystallographic
structures of peptides in order to gain insight in the variability of
the structure when external parameters (pressure, pH,...) are changed.
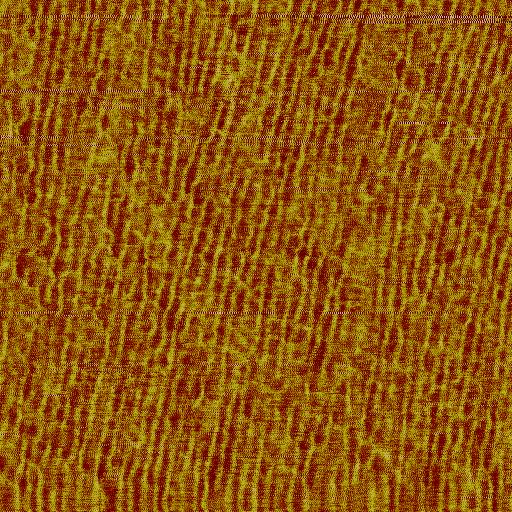
Fig.1 : Phase NC-AFM picture of a deposited peptide layer showing the
alleged perpendicular order to the hydrogen bonds. This kind of
nanostructured pattern is now used for biomimetic mineralization
studies.
Grazing incidence diffraction using synchrotron radiation is one of the
major techniques used for the structural determination of
two-dimensional crystals. Until recently, the resolution of the
technique was limited for crystals of organic molecules at the
air-water or solid-water interface due to the absence of appropriate
methods. We have recently developed a new method, consisting in a
careful extraction of the structure factors from the diffraction data
followed by fitting of molecular parameters which allowed us to reach
near-atomic resolution [2,3]. The structure factor calculations are
performed using the SHELX-97 program and we use the known chemical
structure of the molecules by imposing atomic coordinates in a
molecular model which allows us to fit relevant parameters. Sterically
impossible configurations are automatically rejected by SHELX.
Molecular parameters have a marked influence on the calculated
structure factors and can be determined by model fitting. Using this
method, we could for example evidence a new phase of fatty acids of
symmetry p2gm at high pressure, corresponding to a minimum in
lattice energy, which was never observed. The use of simulated
annealing technique (a Monte-Carlo method) allows a large reduction in
computation time which enables the determination of conformational
defects, whose statistics of conformation are estimated by considering
large super-cells. The same method and the use of Patterson functions
were also used to locate heavy atoms in composite organic-inorganic
two-dimensional crystals. This method can now be extended to even more
complicated cases like peptide or protein two-dimensional crystals
where it could have a major impact.
[1] Lepère M, Chevallard C, Hernandez JF, et al., Multiscale
surface self-assembly of an amyloid-like peptide, LANGMUIR 23 (15):
8150-8155 JUL 17 2007
[2] Pignat, J; Daillant, J; Leiserowitz, L; et al. Grazing incidence
X-ray diffraction on Langmuir films: Toward atomic resolution JOURNAL
OF PHYSICAL CHEMISTRY B, 110 (44): 22178-22184 NOV 9 2006
[3] Pignat, J; Daillant, J; Cantin, S; et al. Grazing incidence X-ray
diffraction study of the tilted phases of Langmuir films: Determination
of molecular conformations using simulated annealing THIN SOLID FILMS,
515 (14): 5691-5695 MAY 23 2007
Contacts : Jean Daillant (jean.daillant@cea.fr); Corinne Chevallard
(corinne.chevallard@cea.fr); Patrick Guenoun (patrick.guenoun@cea.fr).