To overcome some of the limitations of current therapies, new strategies have emerged since the advent of nanotechnology in medicine.
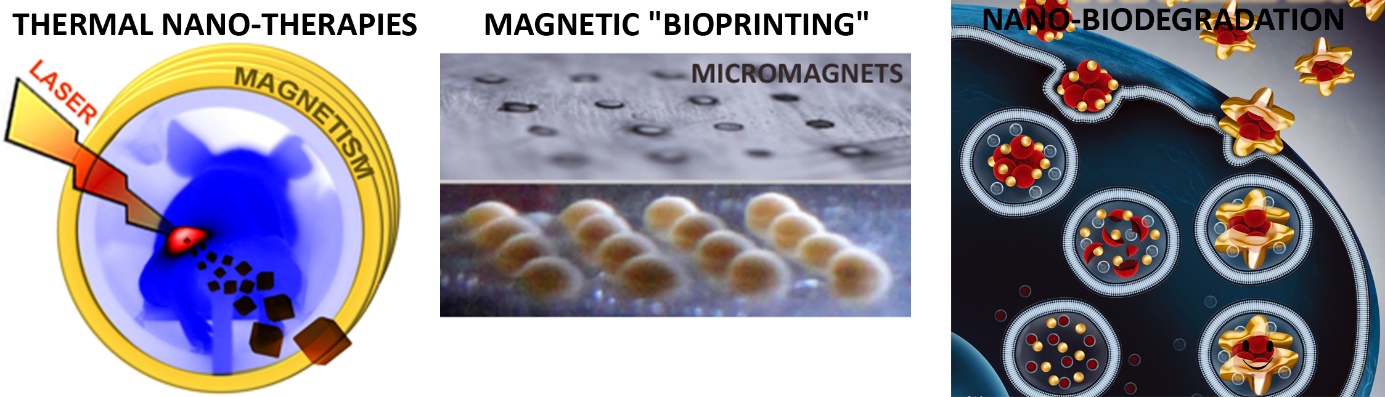
In cancer therapy, thermal treatments (magnetic hyperthermia or photothermal therapy mediated by magnetic or plasmonic nanoparticles) provides noninvasive means of heating and killing cancer cells. In such case, the ultimate target is the cancer cell, so that heating must be generated and measured inside the cells. We provided the first thermal measurements mediated by magnetic [1] or plasmonic [2] nanoparticles inside cancer cells, in vitro or in vivo in the tumor environment, and compared them one to another [3]. The ultimate goal of nanotherapies is anyway to improve the efficacy and combat the tumor from within. We proposed new and combined nanotherapeutic concepts [4-7] based on magneto-photo-thermal stimulation, therapies which led to complete cancer cell destruction in vitro and complete tumor ablation in vivo.
While magnetic nanoparticles are increasingly used as clinical agents for imaging and therapy, their use as a tool for tissue engineering opens up as well challenging perspectives that have rarely been explored. Our strategy has been to take advantage of magnetic nanoparticles internalization to create thick, organized, purely cellular 3D tissue structures [8,9], that can be stimulated on demand [10,11]. This magnetic tissue engineering strategy is an alternative to current popular development of tissue bio-printing.
The use of nanoparticles for cancer cell therapies or tissue engineering raise more general issues of nanoparticles biosafety, once internalized in cells. Yet the nanoparticles long-term tissular fate is poorly documented. We have developed original magnetic and thermal techniques to follow the fate of magnetic and plasmonic nanoparticles and their assimilation within a living tissue, and reveal the massive biotransformations experienced by magnetic nanoparticles [12], together with the remarkable shielding potential of gold shells [13,14].
Bibliography :
