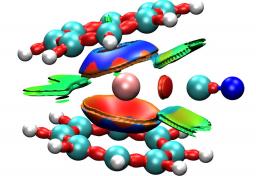
| The chemical bonding in actinide compounds is usually analysed by inspecting the shape and the occupation of the orbitals or by calculating bond orders which are based on orbital overlap and occupation numbers. However, this may not give a definite answer because the choice of the partitioning method may strongly influence the result possibly leading to qualitatively different answers. In this review, we summarized the state-of-the-art of methods dedicated to the theoretical characterisation of bonding including charge, orbital, quantum chemical topology and energy decomposition analyses. This review is not exhaustive but aims to highlight some of the ways opened up by recent methodological developments. Various examples have been chosen to illustrate this progress. |
In this review, Jean-Pierre Dognon summarized the current state-of-the-art of methods dedicated to the theoretical characterisation of bonding. They are well established for organic, inorganic or organometallic compounds. However, some of them cannot be applied to characterise bonding in actinide compounds, but, the recent methodological developments suggest that there will be significant advances in the near future. The f-block still remains a challenge for the theoretical chemist. The very nature of the interaction is far from completely understood. Multiple analysis methods (charge, orbital, quantum chemical topology and energy decomposition analyses) are required to obtain a complete description of bonding. While several simple approaches have been mentioned already, the complexity of the electronic structure of f-element systems makes that the validity of the theoretical models employed must be checked before drawing conclusions.
Understanding covalent mixing in actinide metal–ligand bonds is of particular interest in nuclear sciences and more generally for studies associated with environmental releases of radionuclides and, e.g., to ultimately allow for the design of more selective extractants.
This review is part of a special issue of "Coordination Chemistry Reviews, 50 Years of Progress" : "Chemical bonding in inorganic species: state of the art".
References:
| Electronic structure theory to decipher the chemical bonding in actinide systems, J.-P. Dognon, Coord. Chem. Rev. (2017) |
|
See also : "Une nouvelle approche pour comprendre le comportement chimique des actinides"
"En direct des laboratoires de l'institut de Chimie", CNRS/INC, 9 mai 2017.
"La chimie des actinides, ces métaux radioactifs dont certains sont utilisés en chimie nucléaire, demeure un problème complexe. Spécialiste de la chimie quantique, c’est-à-dire liée au comportement des électrons, Jean-Pierre Dognon du Laboratoire structure et dynamique par résonance magnétique (CNRS/CEA) propose un état de l’art des méthodes récemment développées pour étudier les spécificités des liaisons chimiques formées par les actinides avec d’autres molécules. À la clé : une meilleure connaissance de leur comportement permettant de concevoir ou d’optimiser des systèmes moléculaires aux propriétés physico-chimiques contrôlées. Un travail de synthèse publié dans la revue Coordination Chemistry Reviews".
Contact CEA : Jean-Pierre Dognon (IRAMIS/NIMBE).
•  Interaction laser-matière › Physico-Chimie et Chimie-Physique
Interaction laser-matière › Physico-Chimie et Chimie-Physique  Matériaux des nouvelles technologies pour l’énergie › Chimie des (nano-)matériaux pour l’énergie / Chemistry of (nano-)materials for energy
Matériaux des nouvelles technologies pour l’énergie › Chimie des (nano-)matériaux pour l’énergie / Chemistry of (nano-)materials for energy
•  Institut Rayonnement Matière de Saclay • IRAMIS: Saclay Institute of Matter and Radiation • UMR 3685 NIMBE : Nanosciences et Innovation pour les Matériaux, la Biomédecine et l'Énergie • UMR 3685 NIMBE: Nanoscience and Innovation for Materials, Biomedecine and Energy
Institut Rayonnement Matière de Saclay • IRAMIS: Saclay Institute of Matter and Radiation • UMR 3685 NIMBE : Nanosciences et Innovation pour les Matériaux, la Biomédecine et l'Énergie • UMR 3685 NIMBE: Nanoscience and Innovation for Materials, Biomedecine and Energy
• Laboratoire Structure et Dynamique par Résonance Magnétique (LSDRM)