

IR spectroscopy of jet-cooled short peptide chains allows us to document the network of H-bonds, which is responsible for the stability of the secondary structure of these species, namely their 3-D arrangement.
An important topic in the group aims at collecting accurate structural data in the gas phase to calibrate the modeling of molecular interactions controlling the structure of biomolecules.
Our assets are here the preparation and control of the molecules allowed by gas phase experiments (laser desorption coupled to a supersonic expansion, laser optical spectroscopic methods using several UV and IR lasers) as well as a real interplay between experiment and theory (Modelling of ground state structures and of IR spectra using quantum chemistry, modeling of relaxation processes in supersonic expansions, validation of new theoretical tools for the description of complex systems).
After structural studies of the simplest model peptide chains, which allowed us to characterize the typical secondary structures of proteins (ANR-blanc "LASIHMODo" project - BLAN-08-0158- for "LASer Studies of Isolated and Hydrated peptides for an improved MODelling of proteins"; 2009-2012), the current work is now focused on more complex model peptides and aims at further characterizing the several interactions taking place within a protein, and concerns more particularly the following issues:
i) the influence of hydration on the structural properties, including the flexibility of the peptide backbone;
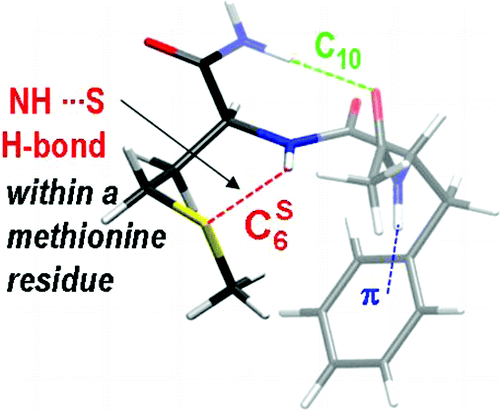
ii) the influence of heteroatomes like sulfur, the interactions of which within proteins are still to poorly documented,
iii) the role of aromatic residues in the spontaneous formation of hydrophobic domains.
•  Physics and chemistry for life sciences and the environment › Physics and life
Physics and chemistry for life sciences and the environment › Physics and life  Laser-matter interaction › Physico-chemistry and Chemical-physics
Laser-matter interaction › Physico-chemistry and Chemical-physics  Laser-matter interaction › Physical Chemistry
Laser-matter interaction › Physical Chemistry
• Interactions, Dynamics and Lasers Laboratory (LIDYL) - CEA-CNRS and Paris Saclay University • Service des Photons Atomes et Molécules • Service des Photons Atomes et Molécules
• Group "BioMolecular Structures" • Laboratoire Francis Perrin











