Crystalline γ-Al2O3 barrier for magnetite-based magnetic tunnel junctions

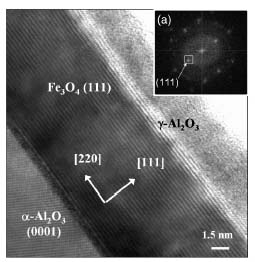
High resolution transmission electron microscopy picture of a Fe3O4/Al2O3 bilayer. Insert: Fourier transform of the picture (coll: P. Bayle-Guillemaud and F. Paumier).
Magnetite is a very attractive oxide for applications in spin electronics devices because this material has been predicted to be an half-metal. In addition the high Curie temperature of this ferrimagnetic compound (Tc = 860 K) makes it possible to hope that the half-metal property persists at room temperature. However, the values of tunnel magneto-resistance (TMR) reported to date are very low at room temperature, except for the junctions using an amorphous alumina barrier for which TMR effects of about 10 % were obtained. The deposition of the alumina barrier is a critical stage, since aluminium has a very strong reactivity with surrounding oxygen. The presence of an alumina layer generally involves the formation of a phase reduced to the Fe3O4/Al2O3 interface, and thus the disappearance of the half-metal behavior of Fe3O4 in the full-course last. In this framework, we carried out a study allowing on the one hand to carry out the epitaxial growth of an alumina layer of some nanometers thickness on magnetite and on the other hand to control the stoichiometry of the Fe3O4/Al2O3 interface. The Fe3O4 (15 nm)/Al2O3 (1.5 nm) bilayers were carried out by MBE. The good epitaxy of the alumina barrier on Fe3O4 is confirmed by the high resolution transmision electron microscopy (HRTEM) picture, showing the crystalline character of alumina deposited.
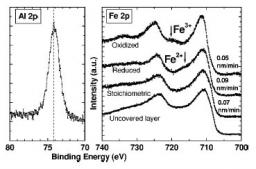
We endeavoured to vary relative oxygen and aluminium fluxes in order to optimize the stoichiometry of magnetite films to the interface. The low thickness of the alumina layer makes it possible to probe the Fe3O4/Al2O3 interface by carrying out XPS spectra at the iron 2p threshold. The presence of multielectronic satellite lines makes it possible to determine the oxidation degree of iron (Fe3+ or Fe2+) and thus qualitatively the stoichiometry of iron oxide films. According to relative values' of oxygen and aluminium fluxes during the deposition, the interface can thus be oxidized, stoechiometric or be reduced (within the limits of the precision of this method). Magnetic measurements (VSM) highlighted the existence of a Verwey transition for the bilayer ones to stoechiometric interface to the same temperature as a single layer of Fe3O4 and to a lower temperature for the bilayer ones to reduced interface. No transition is on the other hand detectable for the bilayer ones with oxidized interface. Being given the extreme sensitivity of the temperature of transition to stoichiometry, we can show from it that the magnetic properties of Fe3O4 are preserved in Fe3O4/Al2O3 stacking at stoechiometric interface, contrary to stackings with oxidized and reduced interface, Appl. Phys. Lett. 86 012509 (2005).
#493 - Last update : 07/18 2005
•  Les archives de l'IRAMIS et du DRECAM / Archives of DRECAM and IRAMIS › Surfaces and nanostructures
Les archives de l'IRAMIS et du DRECAM / Archives of DRECAM and IRAMIS › Surfaces and nanostructures
• Laboratory of Physics and Chemistry of Surfaces and Interfaces