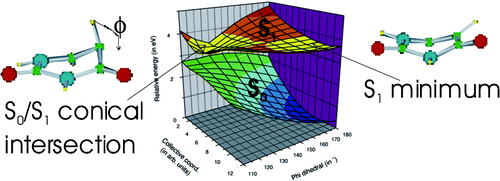
The excited-state properties of uracil, thymine, and nine other derivatives of uracil have been studied by steady-state and time-resolved spectroscopy. The excited-state lifetimes were measured using femtosecond fluorescence upconversion in the UV. The absorption and emission spectra of five representative compounds have been computed at the TD−DFT level, using the PBE0 exchange-correlation functional for ground- and excited-state geometry optimization and the Polarizable Continuum Model (PCM) to simulate the aqueous solution. The calculated spectra are in good agreement with the experimental ones. Experiments show that the excited-state lifetimes of all the compounds examined are dominated by an ultrafast (<100 fs) component. Only 5-substituted compounds show more complex behavior than uracil, exhibiting longer excited-state lifetimes and biexponential fluorescence decays. The S0/S1 conical intersection, located at CASSCF (8/8) level, is indeed characterized by pyramidalization and out of plane motion of the substituents on the C5 atom. A thorough analysis of the excited-state Potential Energy Surfaces, performed at the PCM/TD−DFT(PBE0) level in aqueous solution, shows that the energy barrier separating the local S1 minimum from the conical intersection increases going from uracil through thymine to 5-fluorouracil, in agreement with the ordering of the experimental excited-state lifetime.
•  Physics and chemistry for life sciences and the environment › Physics and life
Physics and chemistry for life sciences and the environment › Physics and life
• Service des Photons Atomes et Molécules • Service des Photons Atomes et Molécules