


 |
 |
 |
Synthesis of nanoparticles [ 21,22,23, 24, 26, 27, 28,29,30] |
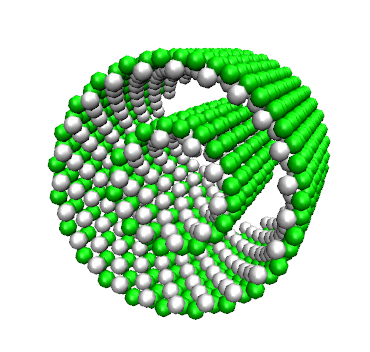 New! AlGe-imogolite-like nanotubes can be either single or double-walled. See more here |
Using high resolution cryo-TEM and Small Angle X-ray Scattering, we have also unravel their mesoscale structure in two contrasted situations. On the one hand, Al-Ge imogolite-like nanotubes synthesized at 0.25M are double-walled nanotubes of 4.0 ± 0.1 nm with an inner tube of 2.4 ± 0.1 nm. Moreover, SAXS data also suggest that the two concentric tubes have an equal length and identical wall structure. On the other hand, at higher concentration (0.5M), both SAXS and cryo-TEM data confirm the formation of single-walled nanotubes of 3.5 ± 0.15 nm. Infrared spectroscopy confirms the imogolite structure of the tubes. This is the first evidence of any double-walled imogolite or imogolite-like nanotubes likely to renew interest in these materials and associated potential applications. |
|
|
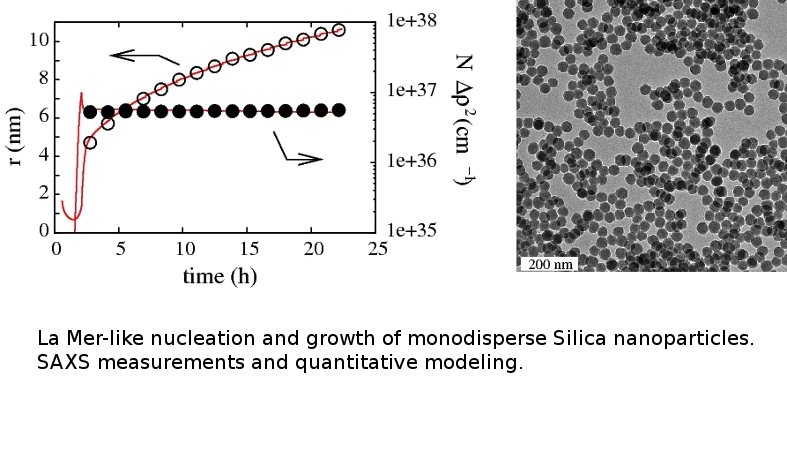 Time resolved SAXS experiment at Soleil (November 2009) |
Toxicity of manufactured nanoparticles [15, 16,17,19, 20, 25 ]: |
 EXAFS measurements at the Ce LIII edge on BM30B beamline at ESRF (avril 2006) |
Thanks to their particular or enhanced properties due to their size, the nanomaterials (dimension < 100 nm) are widely used in many industrial applications (daily care products, nanostructured materials...). However, their growing use together with the belief of easy dispersability frightens because of their uncertain impact on humans and environment. This study is dedicated to a deep understanding of the physicochemical and biological interactions between two cellular models from the environment: Synechocystis (cyanobacteria essential for the biosphere) and Escherichia coli (bacteria of mammalian intestines) with cerium oxide nanoparticles (ex: diesel additive). The particular behavior of nanoparticles (agregation, dissolution, adsorption...) requires a different and highly multidisciplinary approach compared to the study involving classic chemical compounds. Indeed, we showed that the physicochemical parameters (stability, aggregation, dissolution and surface chemistry) of nanoparticles in the contact medium, strongly influence the toxicity [15]. Furthermore, the physicochemical interactions (flocculation, adsorption, redox mechanisms) are linked to the biological model and especially the presence of exopolysaccharides (for Synechocystis) as natural barrier between the cell wall and the nanoparticles. Moreover, the composition of the nanoparticles dispersion medium (particularly the pH) has a major influence on the toxicity (survival and membrane integrity), whereas for E. coli, the nanoparticles are the main culprits for the mortality (confirmed by metabonomic studies via NMR). Read more on the iCEINT web page or phD of Ophélie Zeyons (in french). |
|
It is well known from people doing TEM observations that the aggregation state of the colloids observed after drying is essentially due to drying and does not reflect the "wet" structure. A method to demonstrate this using a custom light scattering apparatus has been published. The modification of the structure of adsorbed colloids during drying is controlled by the competition between adhesion and capillary forces. The drying of nanoparticles confined in spray droplets is also studied. Models for the quantitative analysis of Small Angle Xray Scattering data has been developed. A custom spray dryer has been built to study the drying in situ with small angle X-ray scattering. This work has been presented in the ESRF highlight 2007. read more... |
In Situ Spray Drying experiment at ID02 ESRF. |
Aggregation and structure of aggregates [1, 2, 3, 4, 5, 6, 7, 8, 12]: |
Development of a method using confocal scanning microscopy or light scattering to estimate the fractal dimension of colloids aggregates. This information is used in numerical models to predict the aggregation kinetic of colloids.
This work is a part of my phD thesis available here (in French)