NEW! Internship Position (French M2 level) Open
Together with the researchers from LEDNA/IRAMIS, we are seeking a motivated candidate for an 4-5 months internship project on:
Thermally Charging Ionic SuperCapacitor with Vertically Aligned Carbon NanoTube Electrodes
Interested candidates are invited to read the project description (click on the title above) and contact: sawako.nakamae@cea.fr
Concept
The thermoelectric effect (the Seebeck effect) describes a material’s intrinsic property to directly convert temperature difference (ΔT) applied across its body into electric voltage (ΔV) and vice-versa;
ΔV = -SeΔT (1),
where Se is “the Seebeck coefficient.” TE-generators can be used to recover waste-heat from various sources; e.g., body heat, car exhausts, industrial waste streams, solar heating, etc., to generate electricity. Conversely, the application of voltage ΔV results in a temperature difference (the Peltier effect) such that the same material can be used as a TE-cooler.
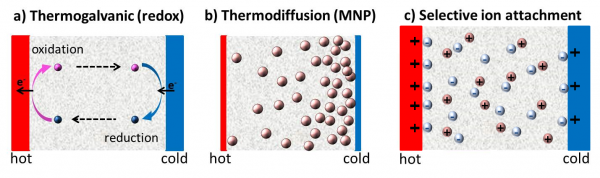
The thermoelectric effect also exists in liquids, although the physical origins of induced electrical potential are quite different. Unlike in solid TE materials, where either the electrons or the holes carry electricity, there are multiple number of 'charged' species in liquid electrolytes and complex fluids that can act as both charge and heat carriers. The thermoelectric voltage and current generation in complex fluids (such as charged colloidal suspensions, ionic liquids, etc.) can be attributed to 3 main physical phenomena; which are; a) Thermogalvanic effect b) thermodiffusion of charged species (ions and/or charged molecules and particles) and c) Selective ion attachments of ionic species (electronic double layer) to the hot and cold electrodes (see figure below).
- Thermogalvanic effect: Today, the thermoelectric applications of liquid electrolytes are predominantly focused on thermogalvanic cells, also known as ‘thermoelectrochemical cells,’ or simply ‘thermocells,’ where redox molecules reversibly exchange electrons (oxidation-reduction mechanism) with cell electrodes to produce large reaction entropy change leading to a thermogalvanic-voltage.
The most well-known example of thermocells is that of aqueous potassium ferro/ferricyanide redox solutions. Here, the redox molecules reversibly exchange electrons with thermocell’s electrodes to produce a large reaction entropy change leading to a thermovoltage (Eq. 3) of the order 1 mV/K [18], i.e.,
(3),
where ΔSAB is the partial molar entropy change of the redox reaction, n is the number of electrons transferred in the reaction and F is Faraday constant.
- Thermodiffusion effect: The TE-effect in complex liquids (electrolytes, ionic liquids, colloidal solutions etc.) is further coupled to the movement of dissolved charged species (ions, molecules or colloidal particles) and is closely related to the “Soret effect” which describes the concentration gradients, ni induced under a temperature gradient;
(Δni/ni ) = αiΔT (4).
The Soret effect or “thermophoresis” has been extensively studied during the last decades (see for example [19] [20] [21]) and is often used for microfluidic characterization and separation. The Soret coefficient, a, is generally proportional to the entropy transported by the moving molecules or particles (i.e., internal degrees of freedom, large solvation shell, particle/molecule size, etc.). Therefore, large thermoelectric coefficients can be expected in complex liquids exhibiting large values of a. Curiously, the corresponding thermoelectric effect has so far attracted much less attention.
Challenges
Despite the robustness of TE-technology (including long life-time, no moving parts, etc.) and its wide range of applicability (wherever a thermal gradient exists), the TE-generators market has so far been restricted to a small number of technological areas due to their low efficiency. The thermal-to-electrical energy conversion efficiency, h of a given TE device/material is most often expressed as a function of a dimensionless parameter ZT, called “figure of merit”:
ZT = TSe2(σ/κ) (2).
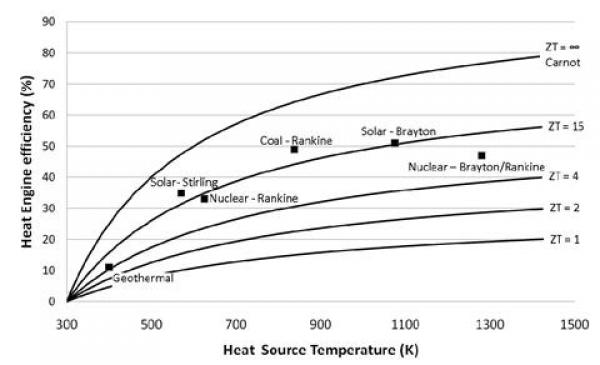
ZT is proportional to the square of the Seebeck coefficient Se and to the ratio between the electrical (σ) and thermal (κ) conductivities. Up until 20 years ago, TE-devices had been based almost exclusively on low-gap solid-state bulk semiconductors whose ZT values are comprised in the ~0.1 – 1.0 range [8]. Compared to other heat engines (see Figure 2.1), there is no wonder why the TE-technology was not considered for a wider and larger-scale heat recovery applications.
TE-coefficients in electrolytes are generally an order of magnitude larger that the semiconductor counterparts, including nanostructured materials. The electrical conductivity of these liquids, however, is a few orders of magnitude lower than solid counterparts and therefore, liquid based TE-systems have long been considered technologically irrelevant despite their advantages; e.g., material abundance, low production costs, etc..
Since the discovery of TE-effects in complex liquids such as ionic-liquids/solvent binary mixtures, and charged colloidal and macromolecular suspensions, such perception surrounding liquid thermoelectrics is changing in recent years. Ionic liquids (IL) are molten salts with low melting temperature (liquid at room temperature), relatively high decomposition temperatures (up to 200°C and higher). ILs’ possess relatively large electrical conductivity values and some have wide electrochemical window [17] making them suitable for variety of low-grade waste heat recovery applications. Charged colloidal and macromolecular suspensions are, as their names suggest, electrolytes containing large carriers of ‘heat’ and ‘electricity’ that contribute to the Seebeck coefficient of the fluid.